We show ~100 events upcoming in the next few months. Here are few recent runners.
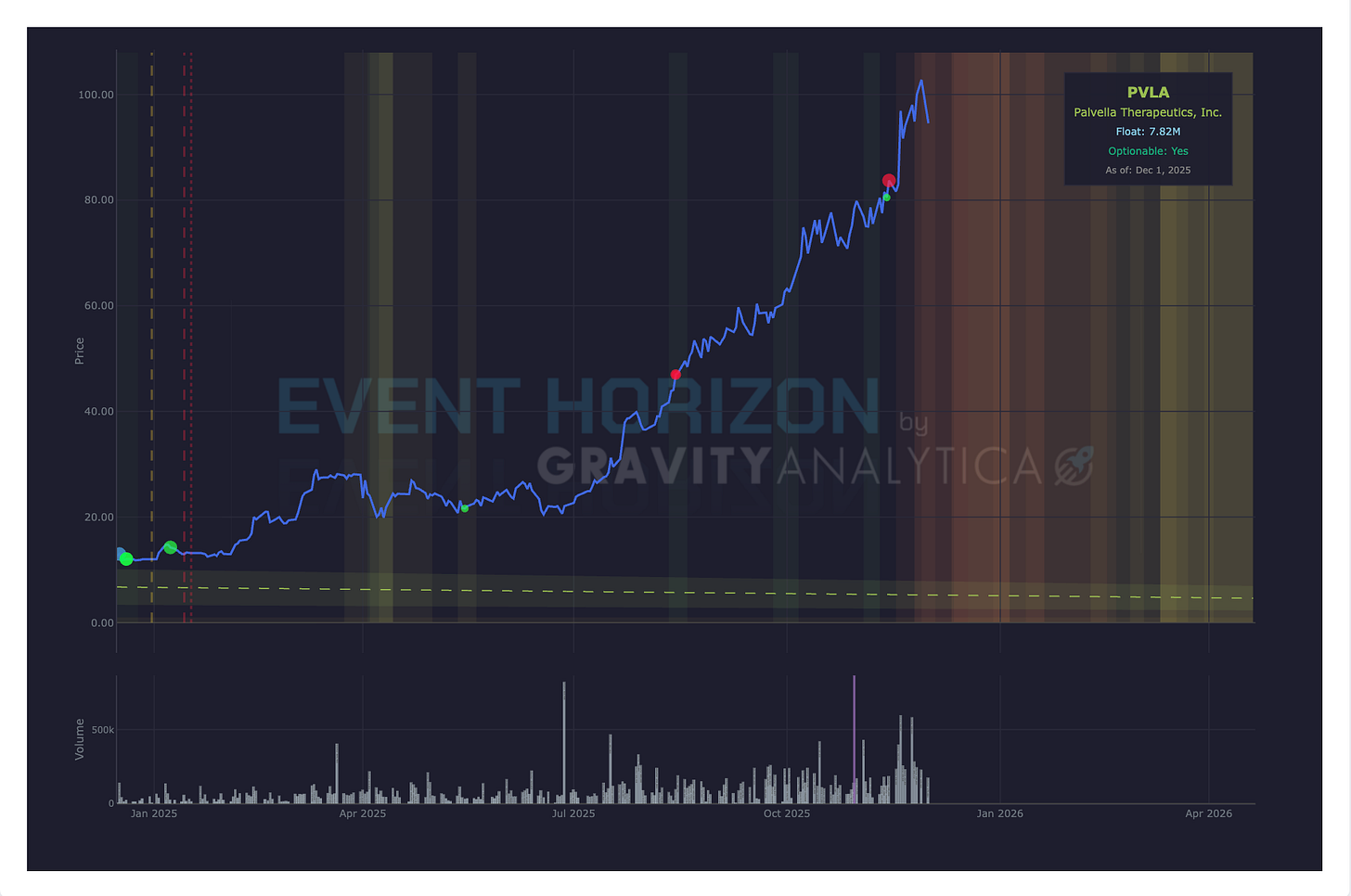
Company: Palvella Therapeutics
1st Data: Top-line Phase II TOIVA
Drug: QTORIN™ 3.9% rapamycin anhydrous gel
Target: Cutaneous venous malformations
Expected: December 2025
2nd Data: Top-line Phase III
Drug: QTORIN™ 3.9% rapamycin anhydrous gel
Target: Microcystic lymphatic malformations
Expected: 1st Quarter 2026
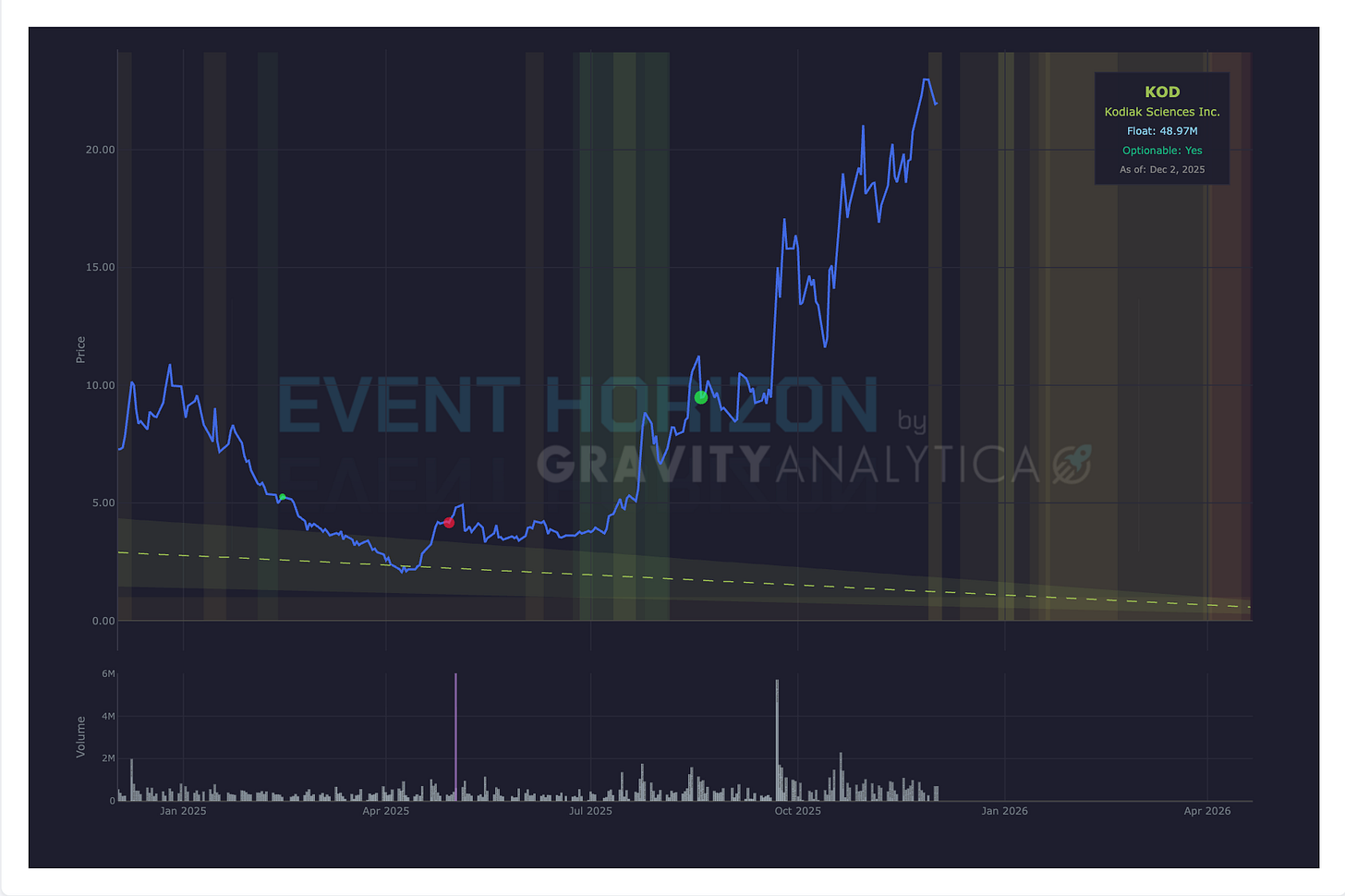
Company: Kodiak Sciences
Data: Top-line Phase III GLOW2
Drug: Tarcocimab
Target: Diabetic retinopathy
Expected: 1st Quarter 2026
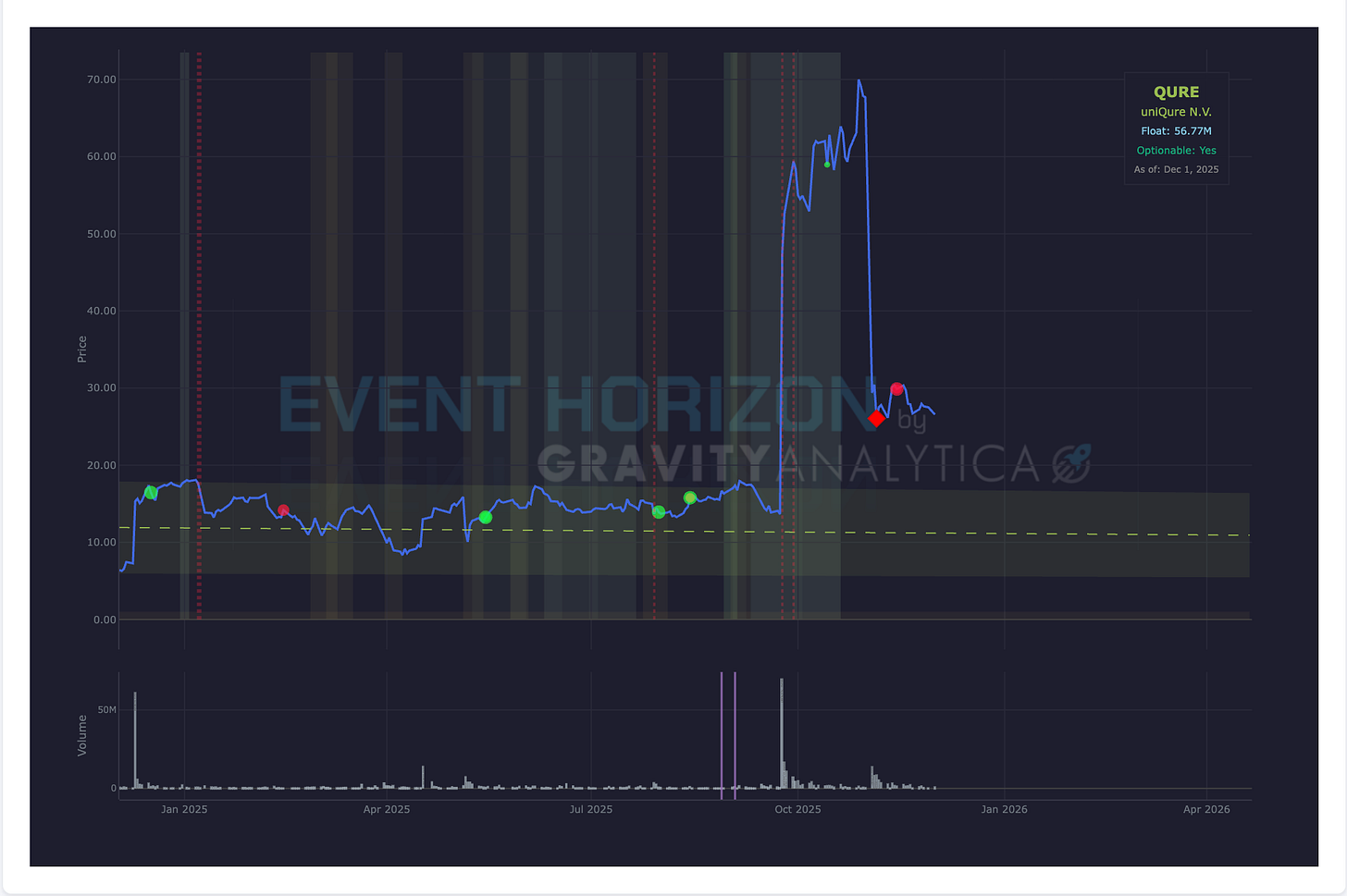
Company: Unicure N.V. (ADR)
Data: N/A, BLA Application
Drug: AMT-130
Target: Huntington's Disease
Expected: 1st Half 2026
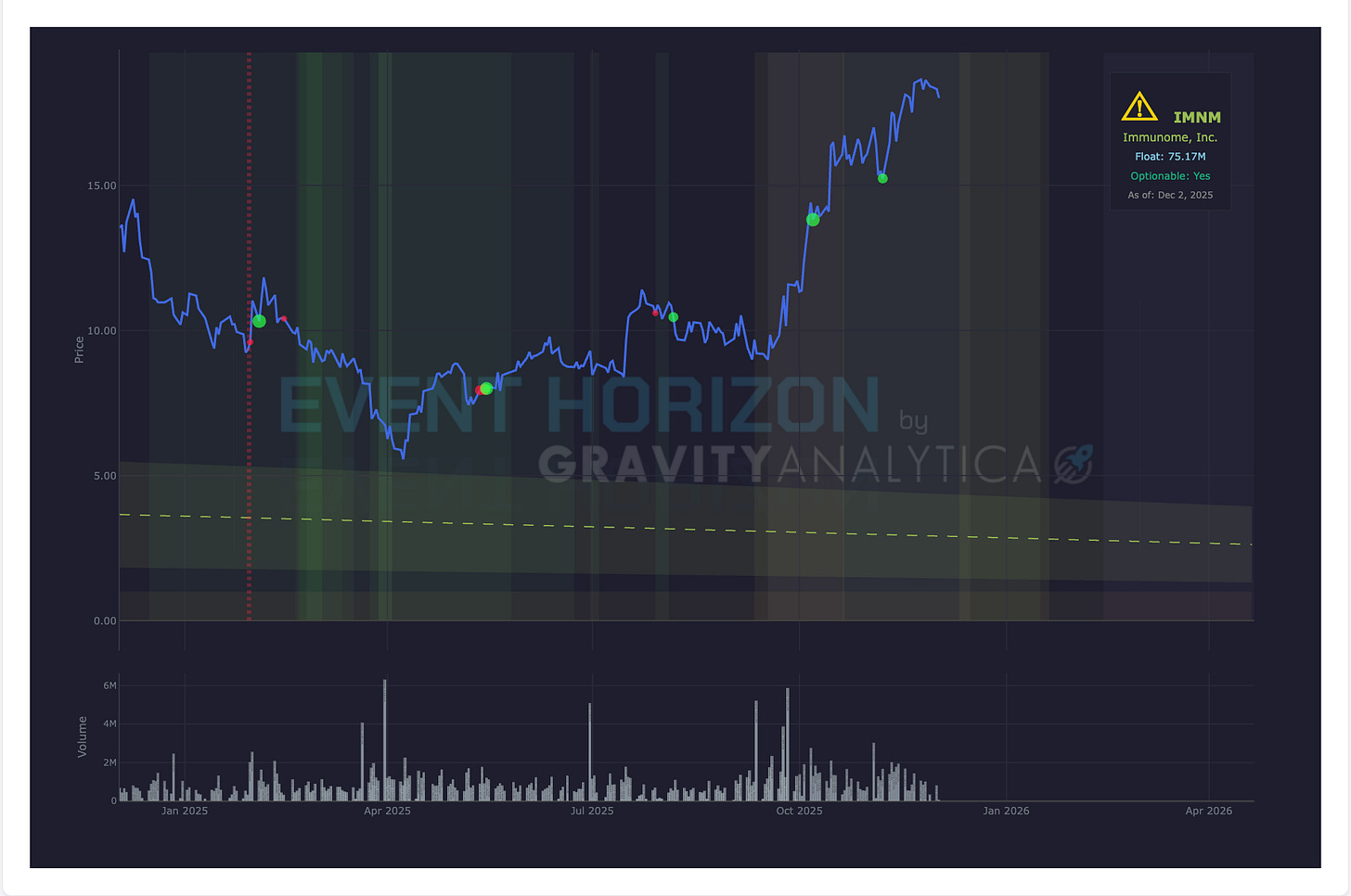
Company: Immunome (Binary)
Data: Top-line Phase III RINGSIDE
Drug: Varegacestat
Target: Desmoid tumors
Expected: December 2025
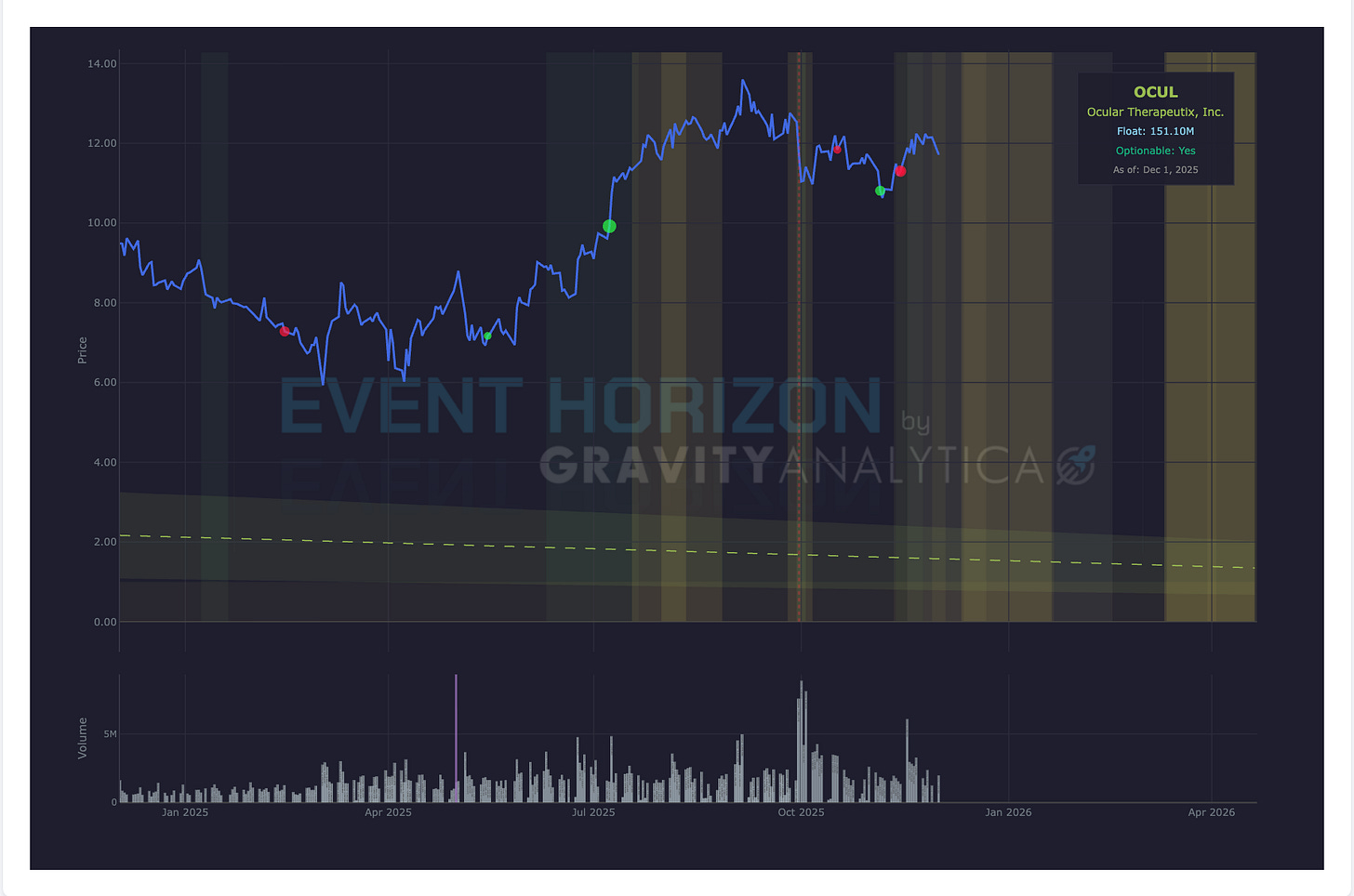
Company: Ocular Therapeutics
Data: Top-line Phase III Sol-1
Drug: AXPAXLI
Target: Wet AMD
Expected: 1st Quarter 2026
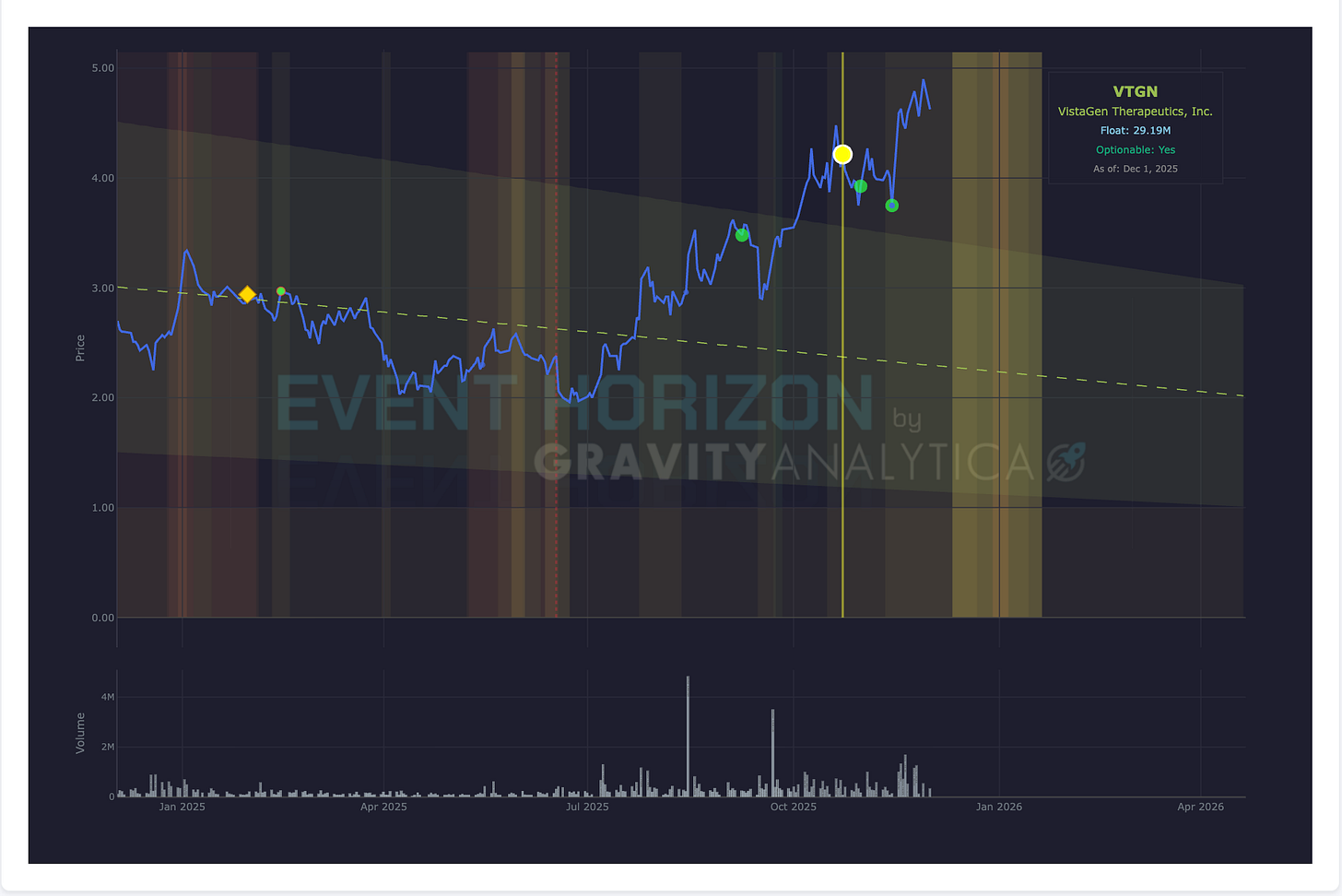
Company: Vistagen Therapeutics
Data: Top-line Phase III PALISADE 3
Drug: Fasedienol
Target: Social Anxiety Disorder
Expected: December 2025
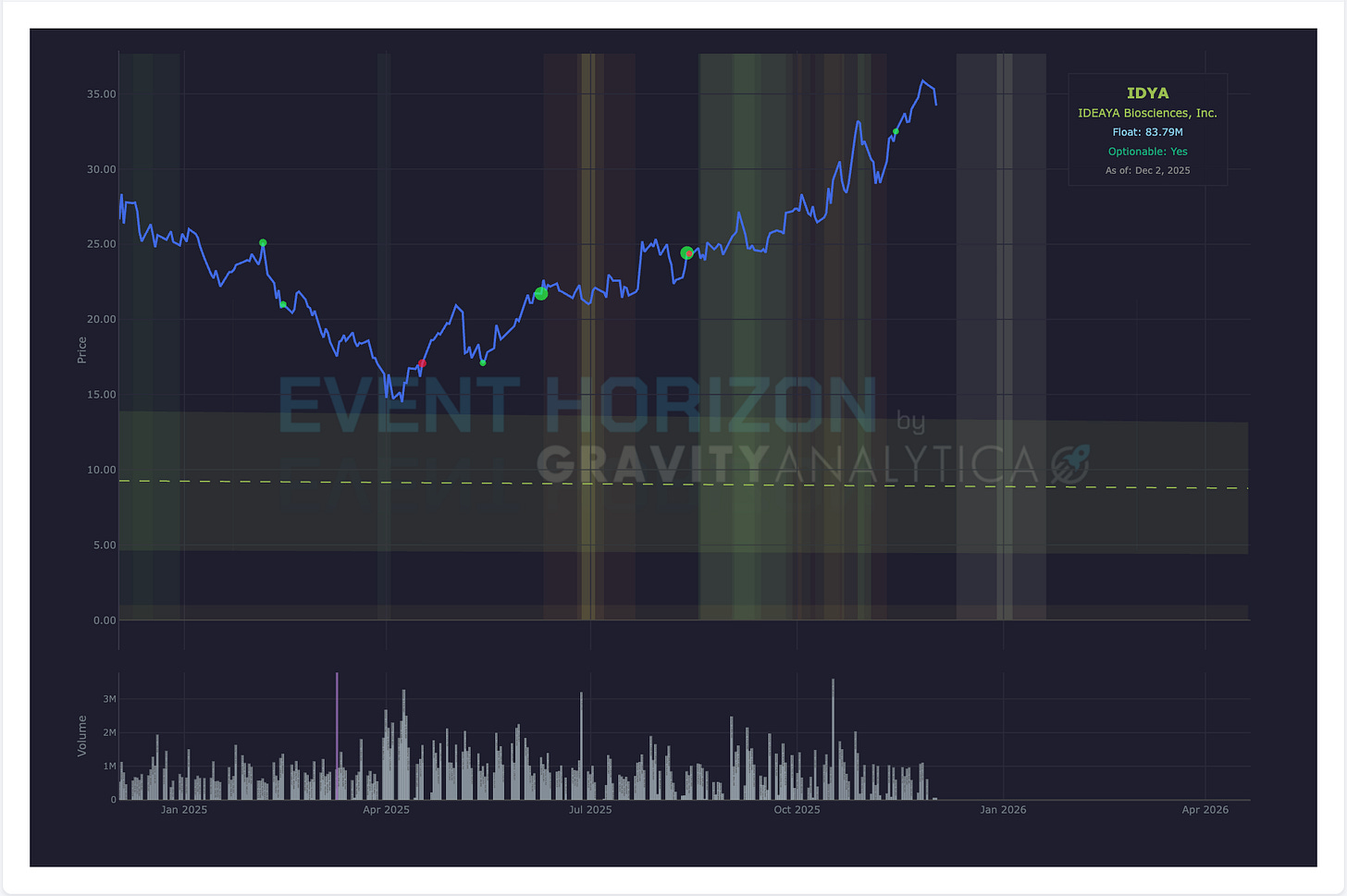
Company: IDEAYA Biosciences (Binary)
Data: Median Phase II/III Combo
Drug: Darovasertib/crizotinib
Target: 1L HLA*A2-negative metastatic uveal melanoma
Expected: December 2025
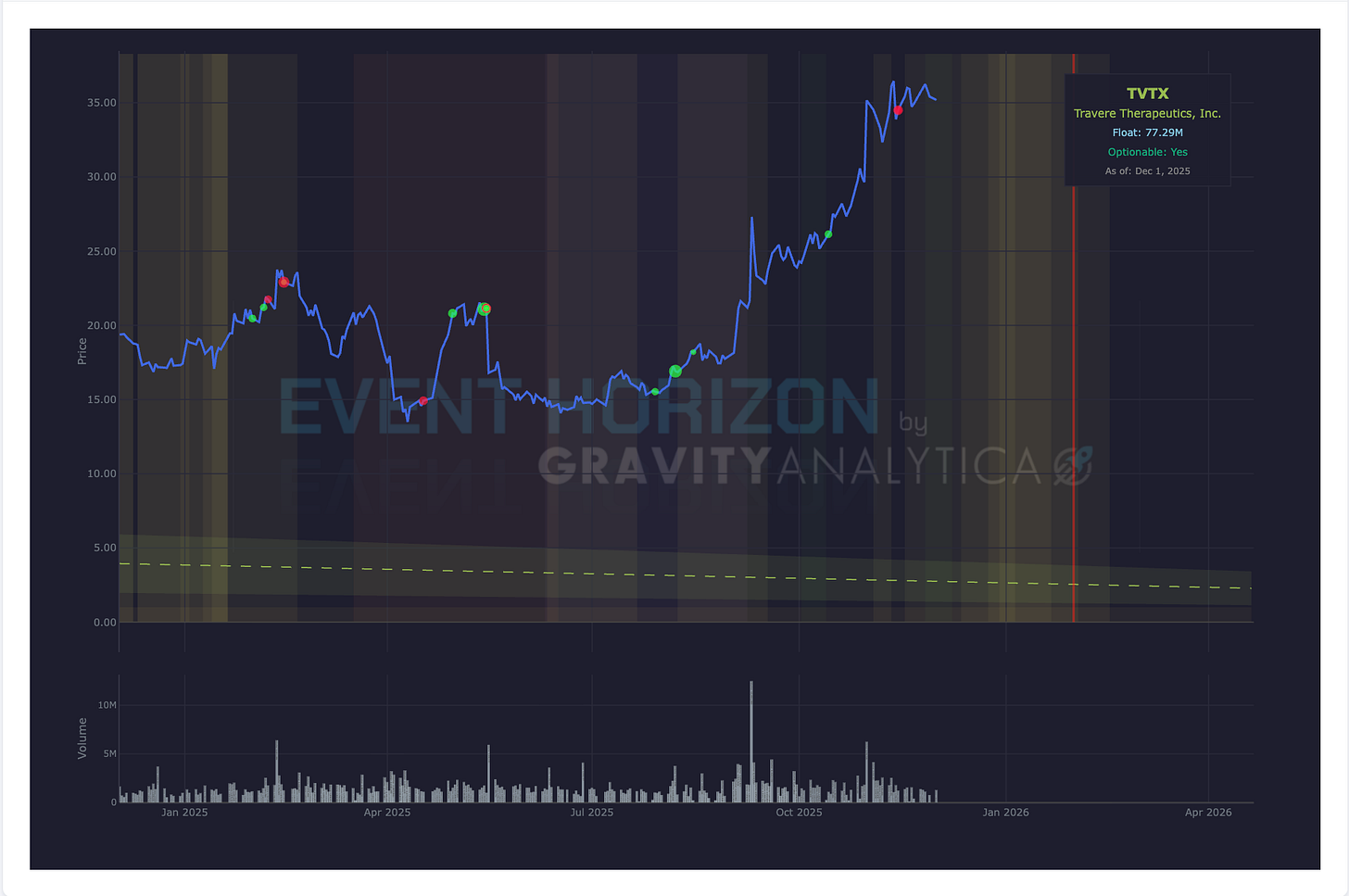
Company: Travere Therapeutics
Data: N/A, PDUFA
Drug: Filspari
Target: Focal segmental glomerulosclerosis
Expected: January 13, 2026
— AJ
Event Horizon Tools
DISCLAIMER: DO NOT BASE ANY INVESTMENT DECISION UPON ANY MATERIALS FOUND ON THIS WEBSITE. We are not registered as a securities broker-dealer or an investment adviser either with the U.S. Securities and Exchange Commission (the “SEC”) or with any state securities regulatory authority. We are neither licensed nor qualified to provide investment advice. Our website has been prepared for informational purposes only and is not intended to be used as a complete source of information on any particular company. Past performance is not indicative of future results. The material contained on this page is intended for informational purposes only. GravityAnalytica.com is wholly-owned by Gravity Analytica, LLC. Our website is not an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content of our website and/or newsletter is not provided to any individual with a view toward their individual circumstances. While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained on our website is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between the any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment. We reserve the right to buy or sell shares of any company mentioned on our website or in our newsletter at any time. We encourage you to invest carefully and read investment information available at the websites of the SEC at http://www.sec.gov and FINRA at http://www.finra.org.
The company or individuals affiliated may hold positions or may enter into, or exit, positions on any equities at any time. This website and materials found on this website, or in any communication, are meant for individuals of eighteen (18) years of age or older and are not suitable for younger audiences. Materials and information provided on this website and in any communications with or from Gravity Analytica LLC, it’s employees or affiliates, are for personal education use by subscribers and may be not be used in any regard in competition with this website or any other product or service offered by Gravity Analytica LLC. Past results do not predict future returns. IF YOU DO NOT AGREE WITH THE TERMS OF THIS DISCLAIMER, PLEASE EXIT THIS SITE IMMEDIATELY. PLEASE BE ADVISED THAT YOUR CONTINUED USE OF THIS SITE OR THE INFORMATION PROVIDED HEREIN SHALL INDICATE YOUR CONSENT AND AGREEMENT TO THESE TERMS.